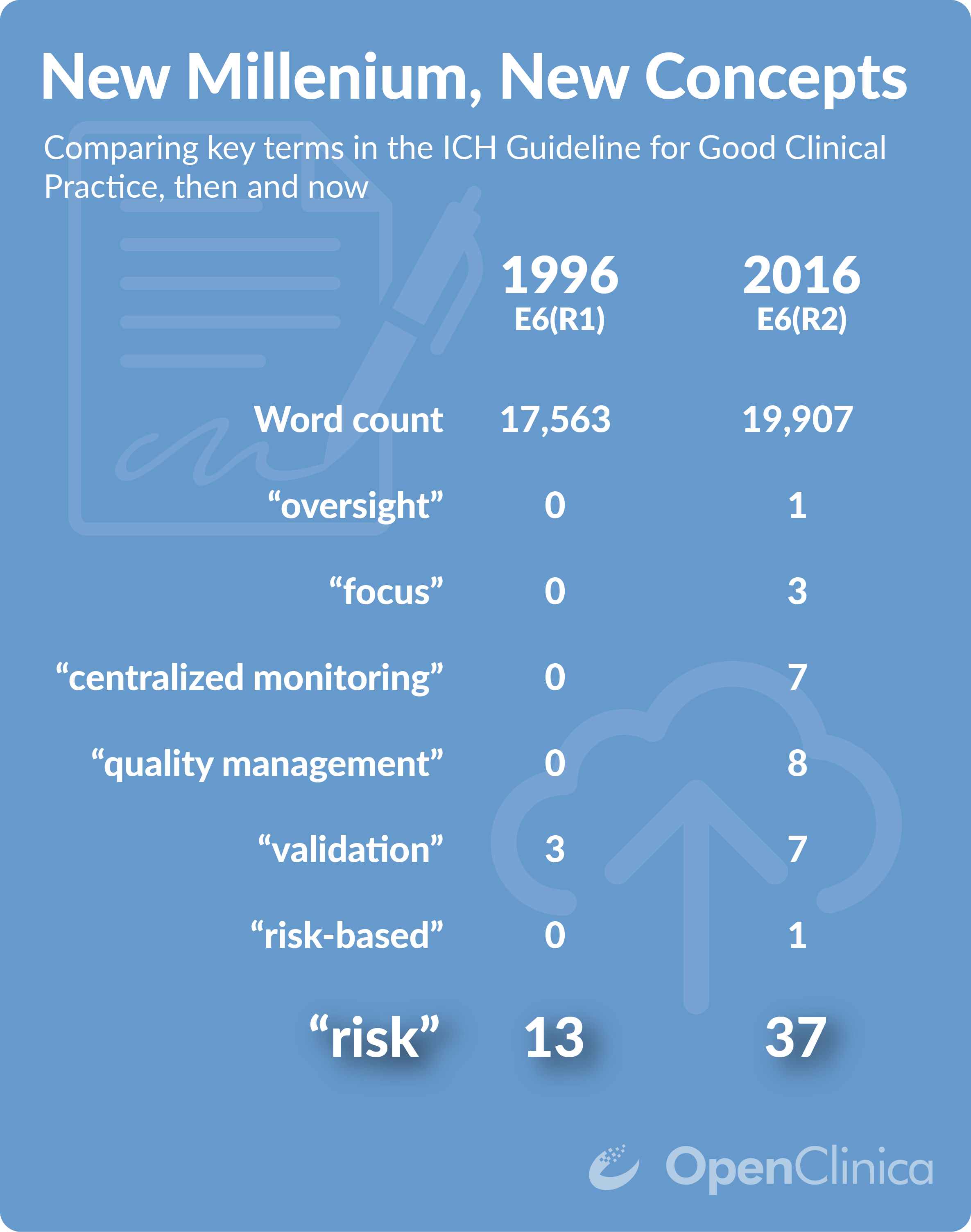
Quanticate launches statistical monitoring services following the ICH GCP E6(R2) addendum | World Pharma Today

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services





%20Guidelines.jpg?width=290&name=Statistical%20Monitoring%20in%20new%20ICH%20GCP%20E6(R2)%20Guidelines.jpg)